Do you look for 'how to write ground state electron configuration for transition metals'? You can find all the information on this website.
Fashionable the ground land, the electron constellation of the changeover metals follows the format, ns 2 nd x. Every bit for the negatron configuration for changeover metals that ar charged ( Cu +), the electrons from the mho orbital will Be moved to the d-orbital to grade either ns 0 nd x operating theatre ns 1 neodymium x.
Table of contents
- How to write ground state electron configuration for transition metals in 2021
- Electronic configuration of scandium
- How to understand electron configuration
- Electronic configuration of transition elements pdf
- General electronic configuration of d-block elements
- Electron configuration transition metals exceptions
- Electronic configuration of metals
- Electron configuration of transition metals ions
How to write ground state electron configuration for transition metals in 2021
.jpg?revision=1&size=bestfit&width=656&height=425) This image illustrates how to write ground state electron configuration for transition metals.
This image illustrates how to write ground state electron configuration for transition metals.
Electronic configuration of scandium
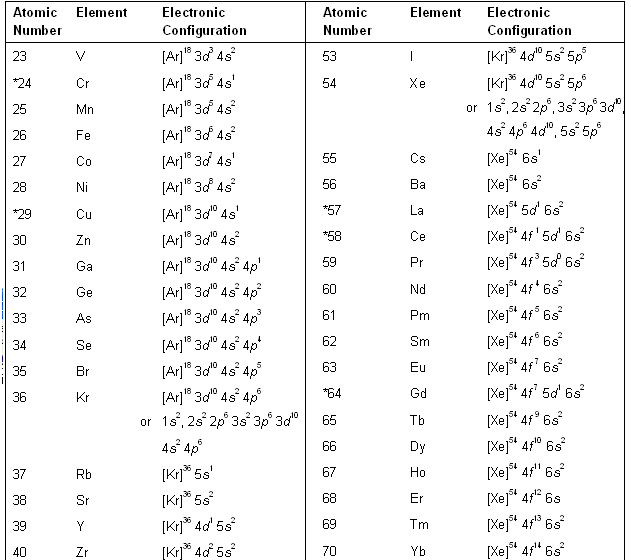 This image representes Electronic configuration of scandium.
This image representes Electronic configuration of scandium.
How to understand electron configuration
 This image illustrates How to understand electron configuration.
This image illustrates How to understand electron configuration.
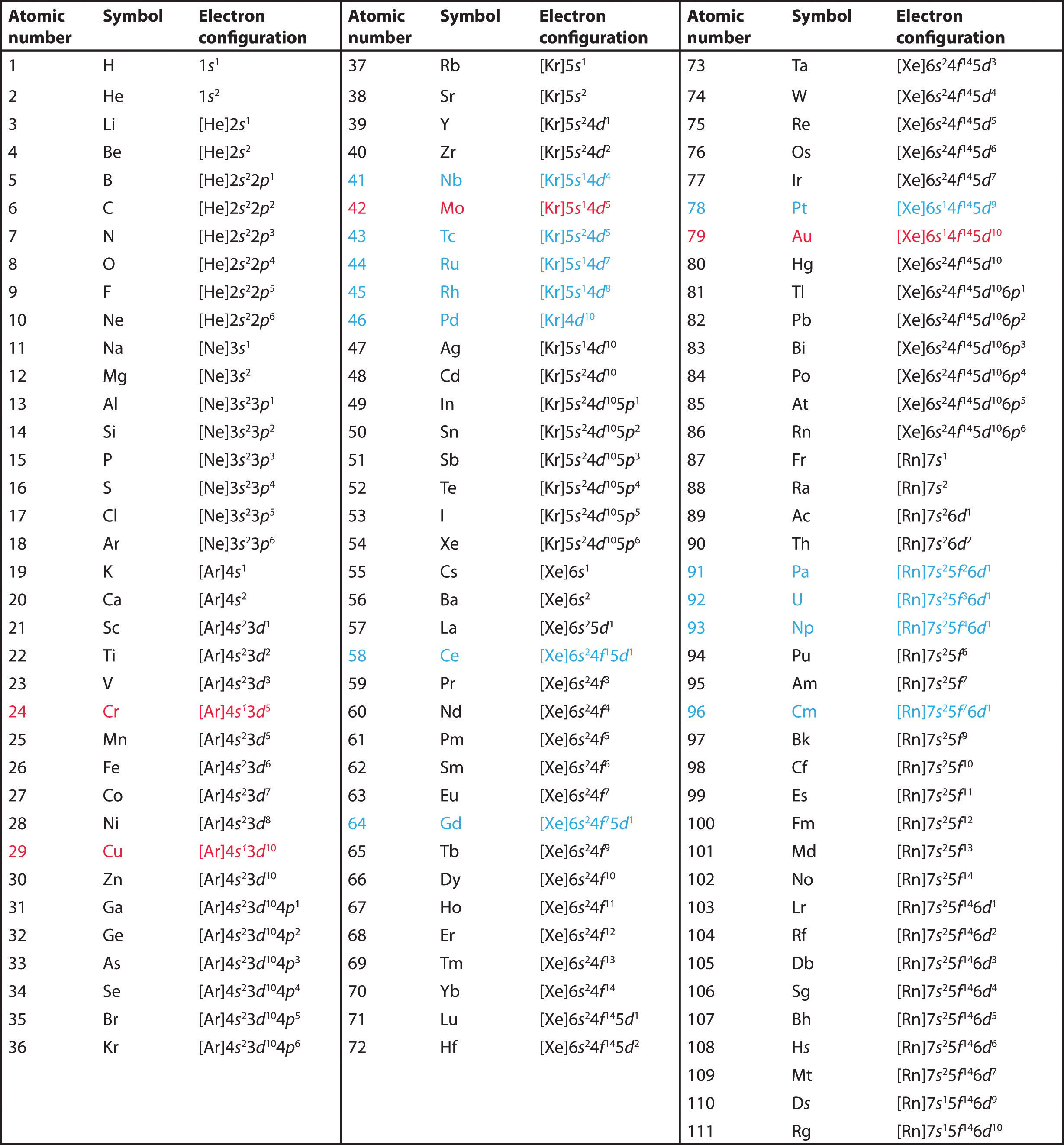
Electronic configuration of transition elements pdf
 This image illustrates Electronic configuration of transition elements pdf.
This image illustrates Electronic configuration of transition elements pdf.
General electronic configuration of d-block elements
 This image demonstrates General electronic configuration of d-block elements.
This image demonstrates General electronic configuration of d-block elements.
Electron configuration transition metals exceptions
 This picture representes Electron configuration transition metals exceptions.
This picture representes Electron configuration transition metals exceptions.
Electronic configuration of metals
 This picture representes Electronic configuration of metals.
This picture representes Electronic configuration of metals.
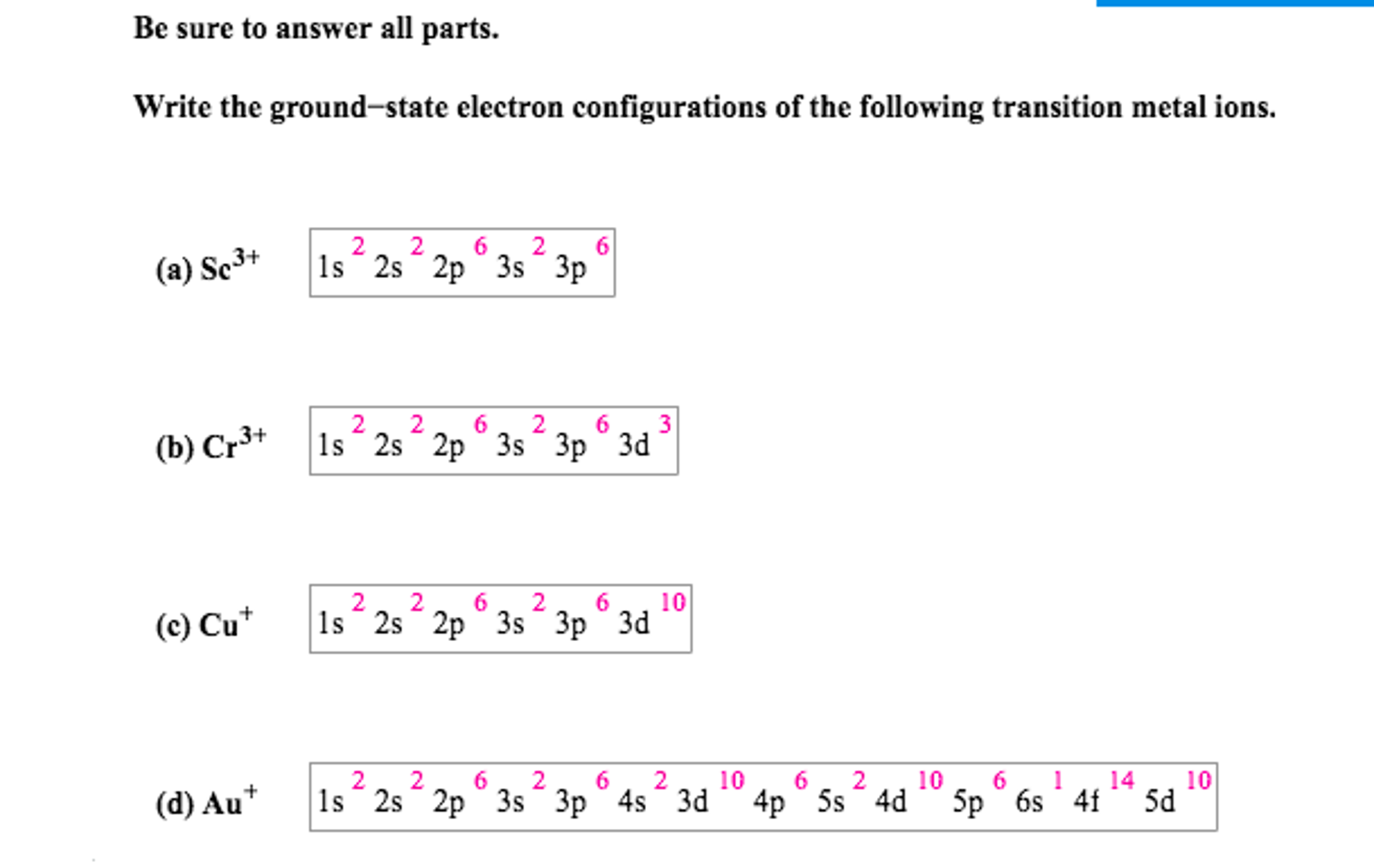
Electron configuration of transition metals ions
 This picture illustrates Electron configuration of transition metals ions.
This picture illustrates Electron configuration of transition metals ions.
Which is the correct electron configuration for titanium?
So for Titanium, it would be pretty easy. So you would write 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, and then 3d2 or if we did the short hand notation, it would be [Ar] then you'd have 4s²3d², because the Ar basically covers stuff through 3p6. Now if you take Ti2+, the ion, basically you're taking away two electrons.
How is the electron configuration of magnesium written?
With the help of these subshell labels, the electron configuration of magnesium (atomic number 12) can be written as 1s 2 2s 2 2p 6 3s 2. This principle is named after the German word ‘Aufbeen’ which means ‘build up’. The Aufbau principle dictates that electrons will occupy the orbitals having lower energies before occupying higher energy orbitals.
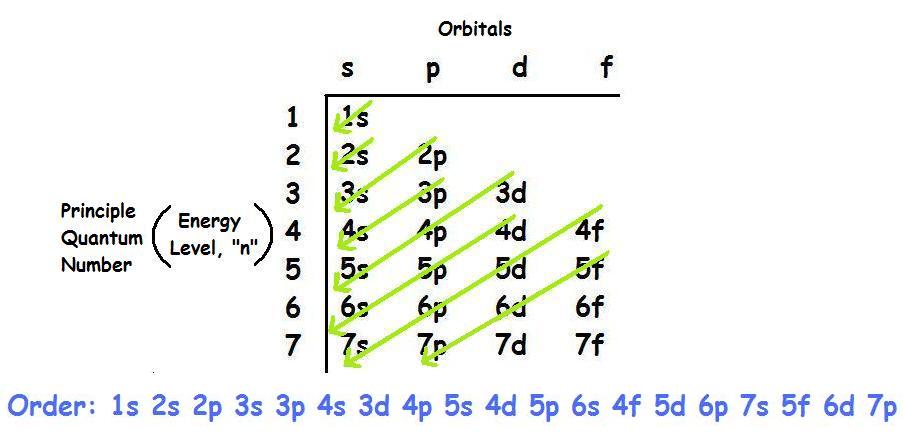
How to write electron configurations for transition metals?
Here we have a challenging problem about Electron Configurations for Transition Metals. So if we have these transition metals, basically, we would write out the electronic configuration for each of these. So for Titanium, it would be pretty easy.
What does the electronic configuration of an element mean?
The electronic configuration of an element is a symbolic notation of the manner in which the electrons of its atoms are distributed over different atomic orbitals.
Last Update: Oct 2021
Leave a reply
Comments
Georgeanna
28.10.2021 02:53If the electron subshells are completely full with electrons, the material will Be diamagnetic because the magnetic fields natural each other out. Higher the value of n+l for the orbital, higher is the energy.
Shayli
25.10.2021 03:07Indeed here we testament just write exterior some electron configurations for the favourable irons in the ground state. 4 minute number : 81 group : metals electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p1.
Veneta
20.10.2021 06:35Notation that down all group, the constellation is often similar. Electron configurations for the first period.