Are you ready to find 'neutralization test for virus'? You will find all the information on this section.
Table of contents
- Neutralization test for virus in 2021
- Virus neutralization test wikipedia
- Neutralization test microbiology
- Virus neutralization test procedure
- Serum virus neutralization assay
- Neutralization test used for
- Surrogate virus neutralization test
- Virus neutralization test protocol
Neutralization test for virus in 2021
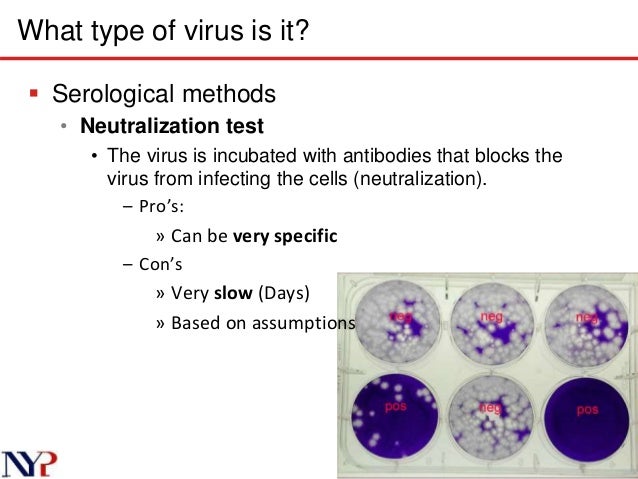 This picture shows neutralization test for virus.
This picture shows neutralization test for virus.
Virus neutralization test wikipedia
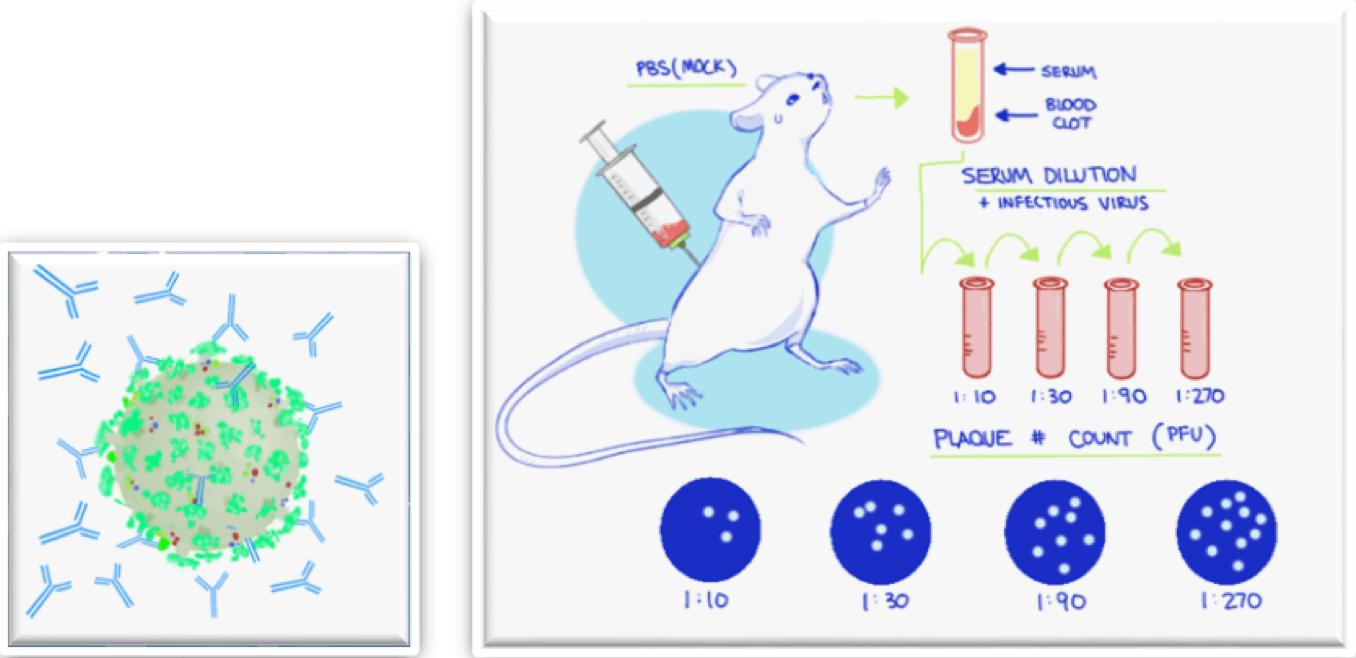 This picture representes Virus neutralization test wikipedia.
This picture representes Virus neutralization test wikipedia.
Neutralization test microbiology
 This picture demonstrates Neutralization test microbiology.
This picture demonstrates Neutralization test microbiology.
Virus neutralization test procedure
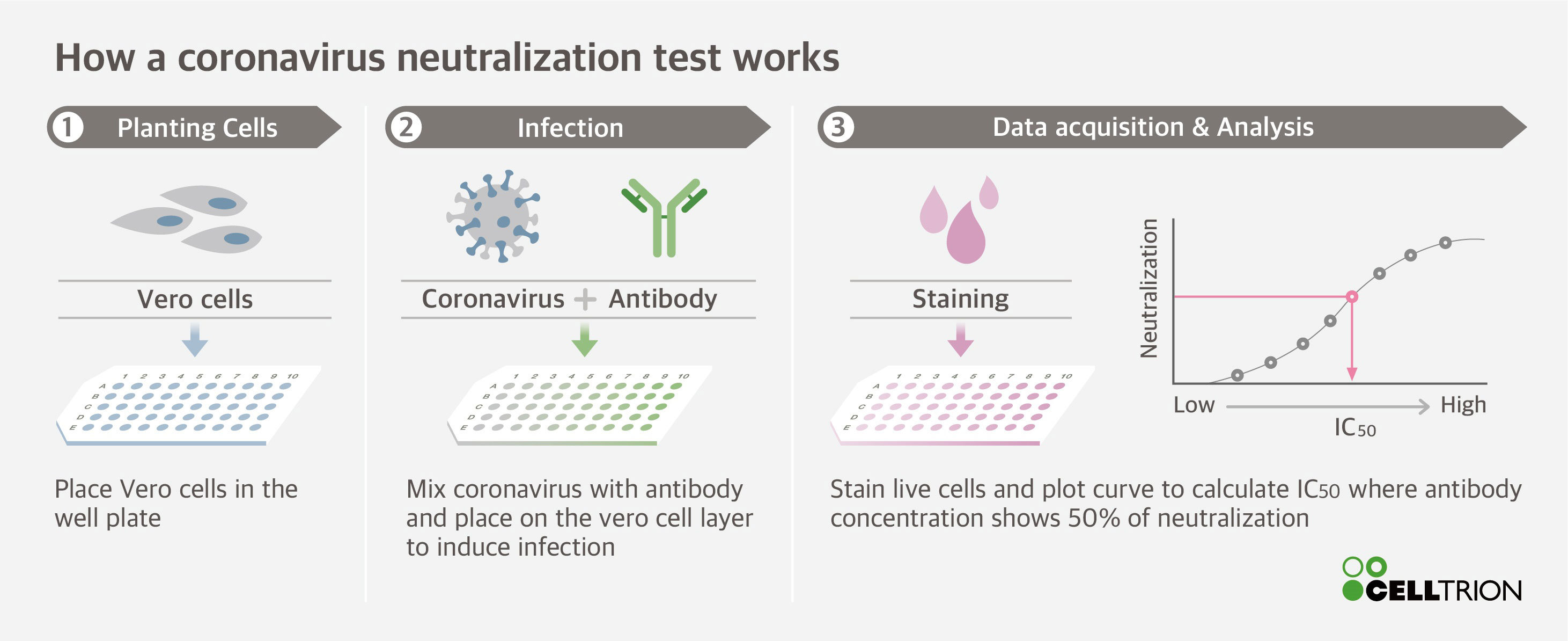 This picture illustrates Virus neutralization test procedure.
This picture illustrates Virus neutralization test procedure.
Serum virus neutralization assay
 This picture shows Serum virus neutralization assay.
This picture shows Serum virus neutralization assay.
Neutralization test used for
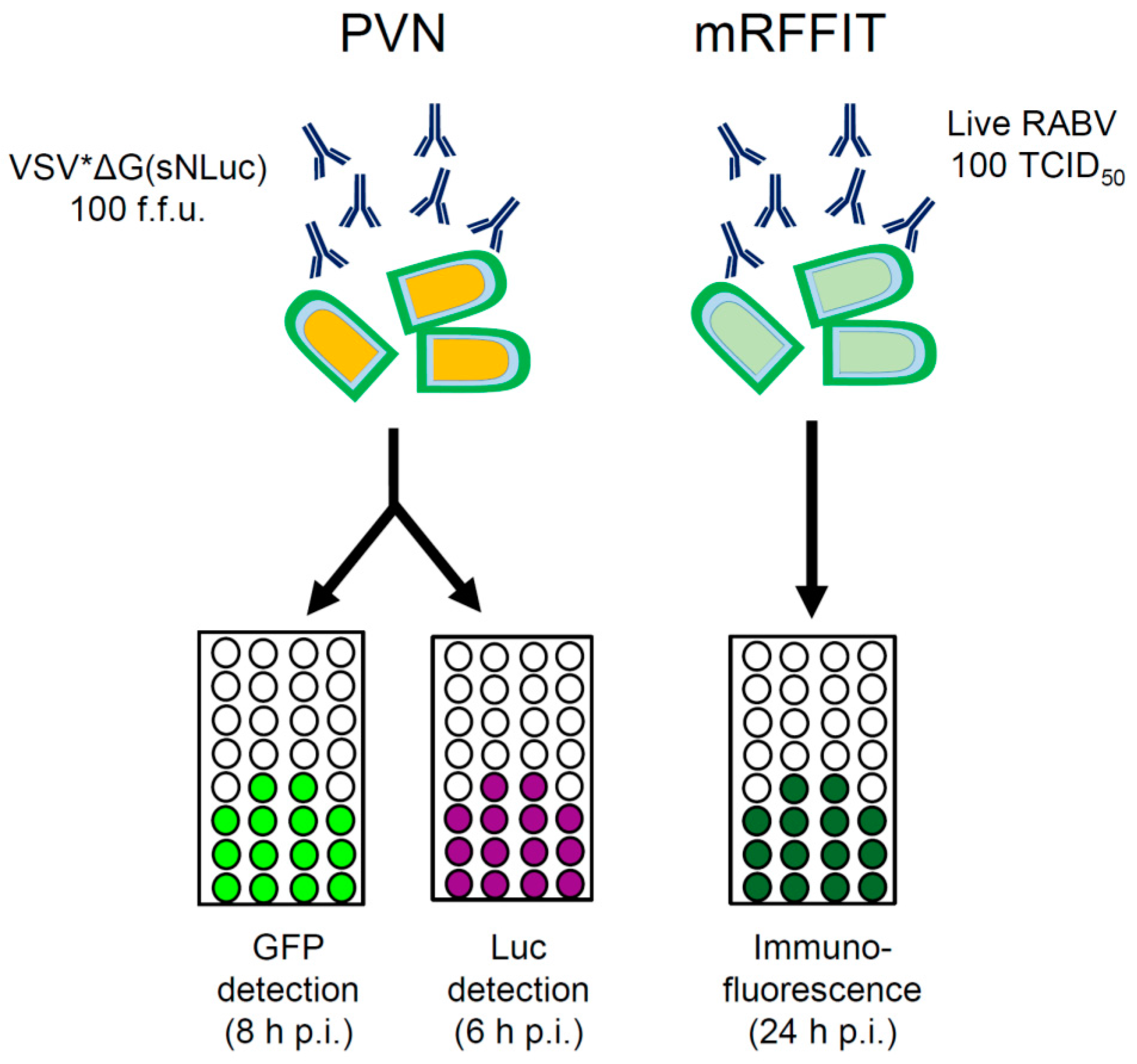 This image illustrates Neutralization test used for.
This image illustrates Neutralization test used for.
Surrogate virus neutralization test
 This picture illustrates Surrogate virus neutralization test.
This picture illustrates Surrogate virus neutralization test.
Virus neutralization test protocol
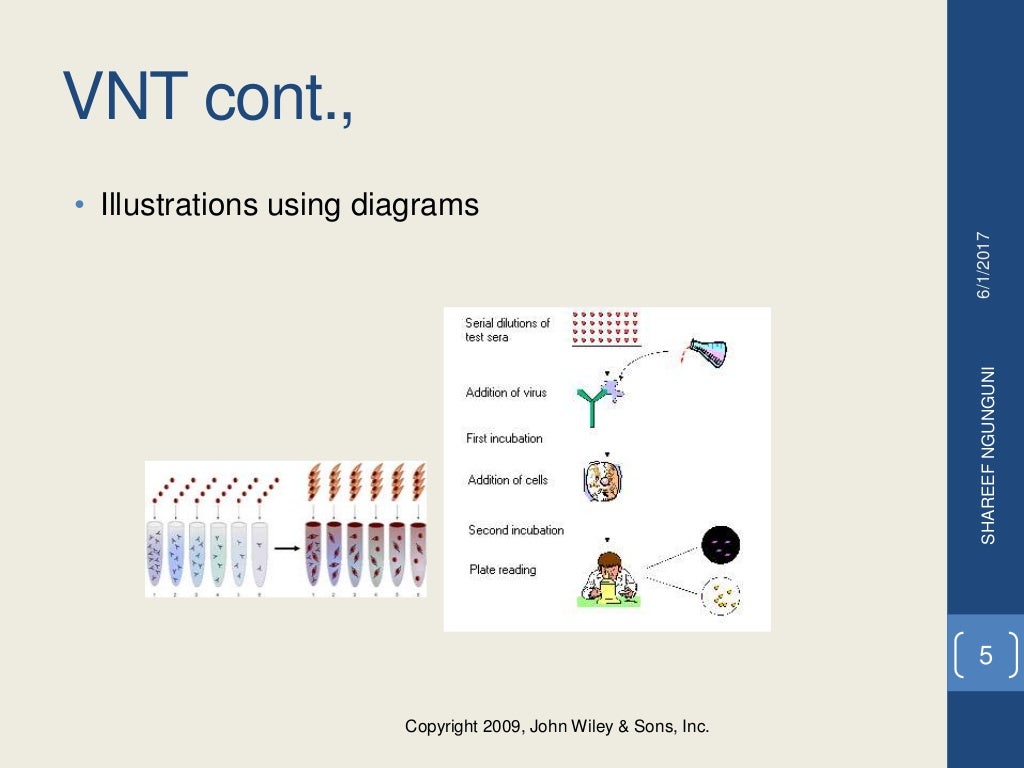 This image shows Virus neutralization test protocol.
This image shows Virus neutralization test protocol.
Which is the best neutralization test for RABV?
The current gold standard RABV neutralization assays, such as rapid fluorescent focus inhibition test (RFFIT) or FAVN, report antibody titers as international units/mL (IU/mL). The PVNA currently only reports antibody titers in reciprocal dilutions.
How are antibodies used in the neutralization test?
Inoculation of viruses in cell cultures, eggs, and animals results in the replication and growth of viruses. When virus-specific neutralizing antibodies are injected into these systems, replication and growth of viruses is inhibited. This forms the basis of virus neutralization test.
What does it mean to neutralize a virus?
Virus neutralization test Neutralization of viruses by their specific antibodies are called virus neutralization tests. Inoculation of viruses in cell cultures, eggs, and animals results in the replication and growth of viruses.
How is antiserum prepared for a neutralization test?
The antiserum is titrated in the neutralization test against its homologous virus. Serial twofold dilutions of serum is prepared and mixed with an equal volume containing 100TCID50 of virus. The virus and serum mixtures are incubated for 1 hour at 37oC.
Last Update: Oct 2021